Background
Nilotinib, a second-generation tyrosine kinase inhibitor (TKI), has been approved for the treatment of patients with newly diagnosed Philadelphia chromosome positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) globally, including Taiwan. However, the real-world evidence regarding the long-term safety (primary) and effectiveness (secondary) of nilotinib in newly diagnosed untreated Ph+ CML-CP is limited in Taiwan.
Methods
The NOVEL-1st study was a non-interventional, multi-center study. A total of 129 patients with newly diagnosed and previously untreated Ph+ CML-CP were enrolled from 11 centers. The objective was to collect the long-term safety (primary) and effectiveness (secondary) data in patients treated with nilotinib under the real clinical practice for up to three years.
Results
Of the 129 enrolled patients, 32 patients (24.8%) discontinued the study, predominately due to AEs (n = 8; 6.2%), discontinuing nilotinib or switching to other treatments (n = 7; 5.4%), and death (n = 5; 3.9%). The median age of the study population was 49 years (range, 20 - 53 years), of whom 77 were male (59.7%). At enrollment, the median time from initial CML diagnosis was 22 days (range, 0.0 - 602 days), and three of them had their CML diagnosed for more than one year.
Across the 3-year observational period, a total of 1,466 AEs were reported in 127 (98.4%) patients, of which 151 AEs in 44 (34.6%) patients were serious adverse events (SAEs) and 524 AEs in 37 (29.1%) patients were nilotinib-related. The most commonly reported non-hematological AEs (regardless of relationship to nilotinib, incidence ≥ 20 %) were rash (24.8%); while that for hematological AEs were thrombocytopenia (31.0%; Grade 3 or above: 13.2%) (Table 1). Nilotinib discontinuation, and dose reduction or interruptions were only reported in 9 (7.1%) and 11 (8.7%) patients, respectively. Five (3.9%) patients expired, of whom 2 were due to CML progression.
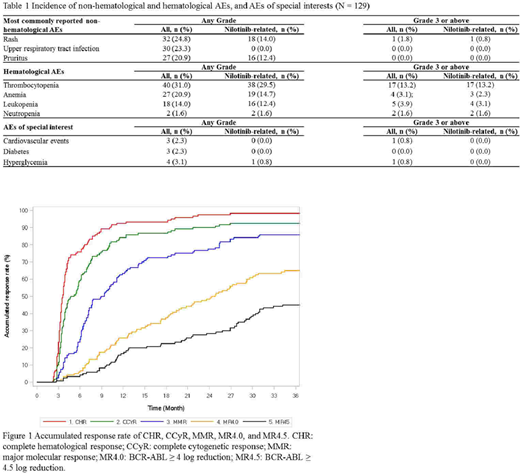
Favorable efficacy outcomes were observed in this real-world study. From 3 to 36 months, the rates of clinical response increased over time, from 72.5% to 98.3% for complete hematological response (CHR), from 48.3% to 92.5% for complete cytogenetic response (CCyR), from 16.7% to 85.8% for major molecular response (MMR), from 4.2% to 65.0% for MR4.0 (BCR-ABL ≥ 4 log reduction), and from 3.3% to 45.0% for MR4.5 (BCR-ABL ≥ 4.5 log reduction) (Figure 1). The median time to CHR, CCyR, MMR, and MR4.0 were 4, 5, 9, and 25 months, respectively (not reached for MR4.5). Early molecular response (EMR), defined as BCR-ABL ≤ 10% at Month 3, was seen in 87.6% of patients.
Non-hematological and hematological AEs were comparable with previous studies, with no new safety signal detected. This NOVEL-1st study demonstrated a lower percentage of patients withdrawing nilotinib owing to AEs (NOVEL-1st vs. prior: 7.1% vs. 10.0%) and an extremely lower incidence of AEs leading to dose reduction or interruption (8.7% vs. 37 - 55%). It was inferred that the high tolerability and patient adherence further contributed to high treatment response in terms of complete molecular response (CMR [i.e., MR4.5], 45% vs. 32%), molecular response 4.0 (MR4.0, 65% vs. 50%), and major molecular response (MMR, 85.5% vs. 73%) at 3 years.
Over the 3 years of follow-up, there was no newly onset of hepatitis or hepatitis B flare. This NOVEL-1st study also reported lower incidence rates of CV events (NOVEL-1st vs. prior: 2.3% vs. 3.5 - 6.0%), diabetes (2.3% vs. 20.2%), and hyperglycemia (3.1% vs. 42.1%) than prior studies. None of them was judged as related to nilotinib treatment (Table 1).
Conclusions
Across the 3-year observational period, nilotinib demonstrated comparable efficacy and had lower risk of hepatitis flare, CV event, diabetes and hyperglycemia among Ph+CML-CP patients in a real world setting in Taiwan. Long-term good patient adherence and holistic care could contribute to good treatment outcome in this chronic disease under the first-line treatment with nilotinib.
Chen:Novartis: Current Employment. Lee:Novartis: Current Employment. Ku:Novartis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract